[vc_row padding_top_multiplier=”” padding_bottom_multiplier=”” columns_gap=”none” equal_column_height=”equal-column”][vc_column vertical_content_position=”middle” width=”5/12″ tablet_sm_width=”1-2″ heading_color=”dark” z_index=”3″][movedo_empty_space][movedo_title heading_tag=”h1″ heading=”h1″ animation=”grve-clipping-animation” animation_delay=”600″ margin_bottom=”0″]iPSC Generation Services[/movedo_title][movedo_title heading_tag=”h1″ heading=”h1″ animation=”grve-clipping-animation” animation_delay=”600″ margin_bottom=”0″]
iPSC Generation On-Demand
[/movedo_title][movedo_empty_space][/vc_column][vc_column width=”7/12″ tablet_sm_width=”1-2″][movedo_single_image image_mode=”medium_large” image=”221″ image_full_column=”yes” image_column_space=”125″ align=”right” animation=”grve-clipping-animation” clipping_animation=”grve-clipping-right”][/vc_column][/vc_row][vc_row bg_type=”color” padding_top_multiplier=”custom” padding_bottom_multiplier=”custom” bg_color=”#2f6197″ padding_top=”10px” padding_bottom=”10px”][vc_column width=”1/3″ heading_color=”light” text_align=”center” css=”.vc_custom_1713722771485{margin-top: 0px !important;margin-bottom: 0px !important;}” font_color=”#ffffff”][movedo_icon_box icon_box_type=”side-icon” icon_size=”small” icon_fontawesome=”far fa-check-circle” icon_color=”white” heading=”h2″]From Human or Non-Human Samples[/movedo_icon_box][/vc_column][vc_column width=”1/3″ heading_color=”light” font_color=”#ffffff”][movedo_icon_box icon_box_type=”side-icon” icon_size=”small” icon_fontawesome=”far fa-check-circle” icon_color=”white” heading=”h2″]Feeder-free, Footprint-free Protocols[/movedo_icon_box][/vc_column][vc_column width=”1/3″ font_color=”#ffffff”][movedo_icon_box icon_box_type=”side-icon” icon_size=”small” icon_fontawesome=”far fa-check-circle” icon_color=”white” heading=”h2″]Research to Clinical Grade[/movedo_icon_box][/vc_column][/vc_row][vc_row][vc_column][vc_column_text css=””]Our iPSC reprogramming service offers a method of generating patient-specific stem cells of any lineage without using embryonic materials. We utilize various strategies to improve reprogramming technologies, including chemical and transgene reprogramming. Our protocols have been streamlined for efficient iPSC generation using viral vectors, DNA (plasmid) and mRNAs. We offer comprehensive reports suitable for publications with each of our services.
We offer reprogramming using various vectors, including retrovirus, lentivirus, episomal plasmid, and direct delivery of synthetic mRNAs. When deciding on a reprogramming method, we consider the cell being reprogrammed and the ability of the reprogramming method to adequately reprogram this cell type. We also assess whether the presence of integrated sequences in the iPSCs will hinder downstream application.
Let the iPSC experts generate high-quality iPSCs and derived physiologically relevant cell line models from your healthy/disease samples. With our optimized reprogramming protocols and comprehensive characterization services, we deliver iPSCs ideal for your basic research, drug discovery, drug screening, and preclinical cell regeneration projects:
- Highly optimized protocols with high reprogramming efficiency (>95% success rate)
- From healthy/diseased human or non-human samples
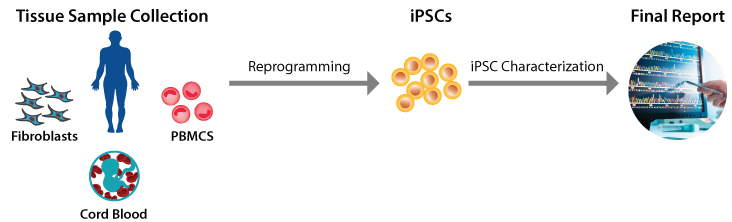
- iPSC generation from various donor cell types
- human: PBMCs, fibroblast, HSC, MSCs, CD34+ cord blood, urine, and more
- non-human: PBMC and fibroblast
- Integration-free (episomal/mRNA/viral-based) or retroviral reprogramming
- Feeder-free protocols; optional feeder-dependent protocols available
- iPSCs characterized for morphology and pluripotency markers. Additional characterization such as G-banding, RT-PCR, STR profiling, and directed differentiation is also available.
- Fast Turnaround: 2-3 months
- GMP iPSC Generation Available
Not only can ASC further characterize your iPSCs, but our experts can also genetically engineer your iPSCs using CRISPR/Cas9 or TARGATT™ and differentiate the iPSCs to the cell type of your choice, including NK cells, T cells, astrocytes, cardiomyocytes, and more.[/vc_column_text][vc_row_inner el_class=”services-button-box” css=”.vc_custom_1714282345231{padding-top: 20px !important;padding-bottom: 20px !important;}”][vc_column_inner][movedo_button align=”inherit” button_text=”Ask an Expert” button_link=”url:https%3A%2F%2Fpinchforthtesting.com%2Fcontact%2F” button_id=”ask-button”][vc_column_text css=””]
Custom iPS Cell Generation
iPSC generation is a complex process that reprograms adult somatic cells into a pluripotent, embryonic stem cell-like stage. iPSCs are typically reprogrammed by introducing products of specific sets of pluripotency-associated genes, or “reprogramming factors”, called Yamanaka factors, into a given cell type. It requires extensive technical expertise to generate cells that are robust, karyotype-normal, and pluripotent. With >15 years of expertise in stem cell technologies and as a leading provider of iPSC services, we can generate iPSCs safely and efficiently in 2-3 months.[/vc_column_text][/vc_column_inner][/vc_row_inner][/vc_column][/vc_row][vc_row el_class=”product-services-box”][vc_column][vc_row_inner][vc_column_inner][vc_custom_heading text=”Services” use_theme_fonts=”yes” css=””][vc_raw_html css=””]JTNDdGFibGUlMjBjbGFzcyUzRCUyMnByb2R1Y3RzLWxpc3QlMjIlM0UlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0aGVhZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RyJTNFJTNDdGglMjBjbGFzcyUzRCUyMmNhdGFsb2clMjIlMjBzY29wZSUzRCUyMmNvbCUyMiUzRUNhdGFsb2clMjBJRCUyMyUzQyUyRnRoJTNFJTBBJTA5JTA5JTNDdGglMjBjbGFzcyUzRCUyMnByb2R1Y3QtbmFtZSUyMiUyMHNjb3BlJTNEJTIyY29sJTIyJTNFU2VydmljZSUyME5hbWUlM0MlMkZ0aCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RoJTIwY2xhc3MlM0QlMjJwcm9kdWN0LXByaWNlJTIyJTIwc2NvcGUlM0QlMjJjb2wlMjIlMjBjb2xzcGFuJTNEJTIyMiUyMiUzRVByaWNlJTNDJTJGdGglM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUzQyUyRnRoZWFkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGJvZHklM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwZGF0YS10aCUzRCUyMkNhdGFsb2clMjBJRCUyMyUyMiUyMGNsYXNzJTNEJTIyY2F0YWxvZyUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2RpdiUyMGNsYXNzJTNEJTIycHJvZHVjdC1pdGVtLW1vZGVsJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDbGFiZWwlM0VBU0MtNjAyMyUzQyUyRmxhYmVsJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDc3BhbiUzRSUyMCUzQyUyRnNwYW4lM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZkaXYlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwZGF0YS10aCUzRCUyMlByb2R1Y3QlMjBOYW1lJTIyJTIwY2xhc3MlM0QlMjJwcm9kdWN0LW5hbWUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NkaXYlMjBjbGFzcyUzRCUyMnByb2R1Y3QlMjBuYW1lJTIwcHJvZHVjdC1pdGVtLW5hbWUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NhJTIwY2xhc3MlM0QlMjJwcm9kdWN0LWl0ZW0tbGluayUyMiUyMGhyZWYlM0QlMjJodHRwcyUzQSUyRiUyRnBpbmNoZm9ydGh0ZXN0aW5nLmNvbSUyRnByb2R1Y3QlMkZ0cmFuc2dlbmljLWFuaW1hbC1mb3ItcHJvdGVpbi1wcm9kdWN0aW9uJTJGJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwaVBTQyUyMEdlbmVyYXRpb24lMjBTZXJ2aWNlJTIwJTI4RXBpc29tYWwlMjklMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZhJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGZGl2JTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGRhdGEtdGglM0QlMjJQcmljZSUyMiUyMGNvbHNwYW4lM0QlMjIyJTIyJTIwY2xhc3MlM0QlMjJwcm9kdWN0LXByaWNlJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDZGl2JTIwY2xhc3MlM0QlMjJwcm9kdWN0LWl0ZW0taW5xdWVyeSUyMGFjdGlvbnMtcHJpbWFyeSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyJTJGY29udGFjdHVzJTJGaW5kZXglMkYlM0ZpZCUzRDkxNiUyNmFtcCUzQmNhdGFsb2clM0RBU0MtNjAyMyUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3NwYW4lM0VJbnF1aXJlJTNDJTJGc3BhbiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZkaXYlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGRhdGEtdGglM0QlMjJDYXRhbG9nJTIwSUQlMjMlMjIlMjBjbGFzcyUzRCUyMmNhdGFsb2clMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NkaXYlMjBjbGFzcyUzRCUyMnByb2R1Y3QtaXRlbS1tb2RlbCUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2xhYmVsJTNFQVNDLTYwMjYlM0MlMkZsYWJlbCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3NwYW4lM0UlMjAlM0MlMkZzcGFuJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGZGl2JTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGRhdGEtdGglM0QlMjJQcm9kdWN0JTIwTmFtZSUyMiUyMGNsYXNzJTNEJTIycHJvZHVjdC1uYW1lJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDZGl2JTIwY2xhc3MlM0QlMjJwcm9kdWN0JTIwbmFtZSUyMHByb2R1Y3QtaXRlbS1uYW1lJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDYSUyMGNsYXNzJTNEJTIycHJvZHVjdC1pdGVtLWxpbmslMjIlMjBocmVmJTNEJTIyaHR0cHMlM0ElMkYlMkZ3d3cuYXBwbGllZHN0ZW1jZWxsLmNvbSUyRnJlc2VhcmNoJTJGc2VydmljZXMlMkZzdGVtLWNlbGwtc2VydmljZXMlMkZzdGVtLWNlbGwtZ2VuZXJhdGlvbiUyRmlwc2MtZ2VuZXJhdGlvbi1ub24taHVtYW4tc3BlY2llcy1yLWFuZC1kJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwaVBTQyUyMEdlbmVyYXRpb24lMjBmcm9tJTIwTm9uLUh1bWFuJTIwU3BlY2llcyUyMCUyOFIlMjZhbXAlM0JEJTI5JTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmRpdiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjBkYXRhLXRoJTNEJTIyUHJpY2UlMjIlMjBjb2xzcGFuJTNEJTIyMiUyMiUyMGNsYXNzJTNEJTIycHJvZHVjdC1wcmljZSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2RpdiUyMGNsYXNzJTNEJTIycHJvZHVjdC1pdGVtLWlucXVlcnklMjBhY3Rpb25zLXByaW1hcnklMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NhJTIwaHJlZiUzRCUyMiUyRmNvbnRhY3R1cyUyRmluZGV4JTJGJTNGaWQlM0Q0NzQzJTI2YW1wJTNCY2F0YWxvZyUzREFTQy02MDI2JTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDc3BhbiUzRUlucXVpcmUlM0MlMkZzcGFuJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmRpdiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwZGF0YS10aCUzRCUyMkNhdGFsb2clMjBJRCUyMyUyMiUyMGNsYXNzJTNEJTIyY2F0YWxvZyUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2RpdiUyMGNsYXNzJTNEJTIycHJvZHVjdC1pdGVtLW1vZGVsJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDbGFiZWwlM0VBU0MtNjAwNyUzQyUyRmxhYmVsJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDc3BhbiUzRSUyMCUzQyUyRnNwYW4lM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZkaXYlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwZGF0YS10aCUzRCUyMlByb2R1Y3QlMjBOYW1lJTIyJTIwY2xhc3MlM0QlMjJwcm9kdWN0LW5hbWUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NkaXYlMjBjbGFzcyUzRCUyMnByb2R1Y3QlMjBuYW1lJTIwcHJvZHVjdC1pdGVtLW5hbWUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NhJTIwY2xhc3MlM0QlMjJwcm9kdWN0LWl0ZW0tbGluayUyMiUyMGhyZWYlM0QlMjJodHRwcyUzQSUyRiUyRnd3dy5hcHBsaWVkc3RlbWNlbGwuY29tJTJGcmVzZWFyY2glMkZzZXJ2aWNlcyUyRnN0ZW0tY2VsbC1zZXJ2aWNlcyUyRnN0ZW0tY2VsbC1nZW5lcmF0aW9uJTJGaHVtYW4tZXMtaXBzLWNlbGwtcGx1cmlwb3RlbmN5LWNoYXJhY3Rlcml6YXRpb24tc2VydmljZS1hc2MtNjAwNyUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMEh1bWFuJTIwRVMlMkZpUFMlMjBDZWxsJTIwUGx1cmlwb3RlbmN5JTIwQ2hhcmFjdGVyaXphdGlvbiUyMFNlcnZpY2UlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZhJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGZGl2JTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGRhdGEtdGglM0QlMjJQcmljZSUyMiUyMGNvbHNwYW4lM0QlMjIyJTIyJTIwY2xhc3MlM0QlMjJwcm9kdWN0LXByaWNlJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDZGl2JTIwY2xhc3MlM0QlMjJwcm9kdWN0LWl0ZW0taW5xdWVyeSUyMGFjdGlvbnMtcHJpbWFyeSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyJTJGY29udGFjdHVzJTJGaW5kZXglMkYlM0ZpZCUzRDg5NCUyNmFtcCUzQmNhdGFsb2clM0RBU0MtNjAwNyUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3NwYW4lM0VJbnF1aXJlJTNDJTJGc3BhbiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZkaXYlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGRhdGEtdGglM0QlMjJDYXRhbG9nJTIwSUQlMjMlMjIlMjBjbGFzcyUzRCUyMmNhdGFsb2clMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NkaXYlMjBjbGFzcyUzRCUyMnByb2R1Y3QtaXRlbS1tb2RlbCUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2xhYmVsJTNFQVNDLTYwMDMtR0glM0MlMkZsYWJlbCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3NwYW4lM0UlMjAlM0MlMkZzcGFuJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGZGl2JTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGRhdGEtdGglM0QlMjJQcm9kdWN0JTIwTmFtZSUyMiUyMGNsYXNzJTNEJTIycHJvZHVjdC1uYW1lJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDZGl2JTIwY2xhc3MlM0QlMjJwcm9kdWN0JTIwbmFtZSUyMHByb2R1Y3QtaXRlbS1uYW1lJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDYSUyMGNsYXNzJTNEJTIycHJvZHVjdC1pdGVtLWxpbmslMjIlMjBocmVmJTNEJTIyaHR0cHMlM0ElMkYlMkZ3d3cuYXBwbGllZHN0ZW1jZWxsLmNvbSUyRnJlc2VhcmNoJTJGc2VydmljZXMlMkZzdGVtLWNlbGwtc2VydmljZXMlMkZzdGVtLWNlbGwtZ2VuZXJhdGlvbiUyRmh1bWFuLWVzLWNlbGwta2FyeW90eXBpbmctc2VydmljZS1nLWJhbmRpbmctaHVtYW4tZXNjLW9yLWlwc2MtYXNjLTYwMDMtZ2glMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjBLYXJ5b3R5cGluZyUyMFNlcnZpY2UlMjAlMjhHLWJhbmRpbmclMjklMjAlMjAtJTIwSHVtYW4lMjBpUFNzJTIwb3IlMjBFU0NzJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmRpdiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjBkYXRhLXRoJTNEJTIyUHJpY2UlMjIlMjBjb2xzcGFuJTNEJTIyMiUyMiUyMGNsYXNzJTNEJTIycHJvZHVjdC1wcmljZSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2RpdiUyMGNsYXNzJTNEJTIycHJvZHVjdC1pdGVtLWlucXVlcnklMjBhY3Rpb25zLXByaW1hcnklMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NhJTIwaHJlZiUzRCUyMiUyRmNvbnRhY3R1cyUyRmluZGV4JTJGJTNGaWQlM0Q4OTAlMjZhbXAlM0JjYXRhbG9nJTNEQVNDLTYwMDMtR0glMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NzcGFuJTNFSW5xdWlyZSUzQyUyRnNwYW4lM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZhJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGZGl2JTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjBkYXRhLXRoJTNEJTIyQ2F0YWxvZyUyMElEJTIzJTIyJTIwY2xhc3MlM0QlMjJjYXRhbG9nJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDZGl2JTIwY2xhc3MlM0QlMjJwcm9kdWN0LWl0ZW0tbW9kZWwlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NsYWJlbCUzRUFTQy02MDAzLUhMQSUzQyUyRmxhYmVsJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDc3BhbiUzRSUyMCUzQyUyRnNwYW4lM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZkaXYlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwZGF0YS10aCUzRCUyMlByb2R1Y3QlMjBOYW1lJTIyJTIwY2xhc3MlM0QlMjJwcm9kdWN0LW5hbWUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NkaXYlMjBjbGFzcyUzRCUyMnByb2R1Y3QlMjBuYW1lJTIwcHJvZHVjdC1pdGVtLW5hbWUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NhJTIwY2xhc3MlM0QlMjJwcm9kdWN0LWl0ZW0tbGluayUyMiUyMGhyZWYlM0QlMjJodHRwcyUzQSUyRiUyRnd3dy5hcHBsaWVkc3RlbWNlbGwuY29tJTJGcmVzZWFyY2glMkZzZXJ2aWNlcyUyRnN0ZW0tY2VsbC1zZXJ2aWNlcyUyRnN0ZW0tY2VsbC1nZW5lcmF0aW9uJTJGaGxhLXR5cGluZy1zZXJ2aWNlLWZyb20taXBzY3MlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjBITEElMjBUeXBpbmclMjBTZXJ2aWNlJTIwJTI4aVBTQ3MlMjklMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZhJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGZGl2JTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGRhdGEtdGglM0QlMjJQcmljZSUyMiUyMGNvbHNwYW4lM0QlMjIyJTIyJTIwY2xhc3MlM0QlMjJwcm9kdWN0LXByaWNlJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDZGl2JTIwY2xhc3MlM0QlMjJwcm9kdWN0LWl0ZW0taW5xdWVyeSUyMGFjdGlvbnMtcHJpbWFyeSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyJTJGY29udGFjdHVzJTJGaW5kZXglMkYlM0ZpZCUzRDQ0MDIlMjZhbXAlM0JjYXRhbG9nJTNEQVNDLTYwMDMtSExBJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDc3BhbiUzRUlucXVpcmUlM0MlMkZzcGFuJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmRpdiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwZGF0YS10aCUzRCUyMkNhdGFsb2clMjBJRCUyMyUyMiUyMGNsYXNzJTNEJTIyY2F0YWxvZyUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2RpdiUyMGNsYXNzJTNEJTIycHJvZHVjdC1pdGVtLW1vZGVsJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDbGFiZWwlM0VBU0MtNjAwOC0xJTNDJTJGbGFiZWwlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NzcGFuJTNFJTIwJTNDJTJGc3BhbiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmRpdiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdGQlMjBkYXRhLXRoJTNEJTIyUHJvZHVjdCUyME5hbWUlMjIlMjBjbGFzcyUzRCUyMnByb2R1Y3QtbmFtZSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2RpdiUyMGNsYXNzJTNEJTIycHJvZHVjdCUyMG5hbWUlMjBwcm9kdWN0LWl0ZW0tbmFtZSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBjbGFzcyUzRCUyMnByb2R1Y3QtaXRlbS1saW5rJTIyJTIwaHJlZiUzRCUyMmh0dHBzJTNBJTJGJTJGcGluY2hmb3J0aHRlc3RpbmcuY29tJTJGcHJvZHVjdCUyRnRlcmF0b21hLWZvcm1hdGlvbi1hbmFseXNpcyUyRiUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMFRlcmF0b21hJTIwRm9ybWF0aW9uJTIwQW5hbHlzaXMlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZhJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGZGl2JTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGRhdGEtdGglM0QlMjJQcmljZSUyMiUyMGNvbHNwYW4lM0QlMjIyJTIyJTIwY2xhc3MlM0QlMjJwcm9kdWN0LXByaWNlJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDZGl2JTIwY2xhc3MlM0QlMjJwcm9kdWN0LWl0ZW0taW5xdWVyeSUyMGFjdGlvbnMtcHJpbWFyeSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyJTJGY29udGFjdHVzJTJGaW5kZXglMkYlM0ZpZCUzRDg5NSUyNmFtcCUzQmNhdGFsb2clM0RBU0MtNjAwOC0xJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDc3BhbiUzRUlucXVpcmUlM0MlMkZzcGFuJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGYSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmRpdiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRkJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ciUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwZGF0YS10aCUzRCUyMkNhdGFsb2clMjBJRCUyMyUyMiUyMGNsYXNzJTNEJTIyY2F0YWxvZyUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2RpdiUyMGNsYXNzJTNEJTIycHJvZHVjdC1pdGVtLW1vZGVsJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDbGFiZWwlM0VBU0MtOTAyMSUzQyUyRmxhYmVsJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDc3BhbiUzRSUyMCUzQyUyRnNwYW4lM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZkaXYlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwZGF0YS10aCUzRCUyMlByb2R1Y3QlMjBOYW1lJTIyJTIwY2xhc3MlM0QlMjJwcm9kdWN0LW5hbWUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NkaXYlMjBjbGFzcyUzRCUyMnByb2R1Y3QlMjBuYW1lJTIwcHJvZHVjdC1pdGVtLW5hbWUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NhJTIwY2xhc3MlM0QlMjJwcm9kdWN0LWl0ZW0tbGluayUyMiUyMGhyZWYlM0QlMjJodHRwcyUzQSUyRiUyRnd3dy5hcHBsaWVkc3RlbWNlbGwuY29tJTJGcmVzZWFyY2glMkZzZXJ2aWNlcyUyRnN0ZW0tY2VsbC1zZXJ2aWNlcyUyRnN0ZW0tY2VsbC1nZW5lcmF0aW9uJTJGYXNjLTkwMjEtdHJpLWxpbmVhZ2UtZGlyZWN0ZWQtZGlmZmVyZW50aWF0aW9uJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwVHJpLWxpbmVhZ2UlMjBEaXJlY3RlZCUyMERpZmZlcmVudGlhdGlvbiUyMFNlcnZpY2UlMjBmcm9tJTIwaVBTQyUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZkaXYlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwZGF0YS10aCUzRCUyMlByaWNlJTIyJTIwY29sc3BhbiUzRCUyMjIlMjIlMjBjbGFzcyUzRCUyMnByb2R1Y3QtcHJpY2UlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NkaXYlMjBjbGFzcyUzRCUyMnByb2R1Y3QtaXRlbS1pbnF1ZXJ5JTIwYWN0aW9ucy1wcmltYXJ5JTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDYSUyMGhyZWYlM0QlMjIlMkZjb250YWN0dXMlMkZpbmRleCUyRiUzRmlkJTNENDI4MiUyNmFtcCUzQmNhdGFsb2clM0RBU0MtOTAyMSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3NwYW4lM0VJbnF1aXJlJTNDJTJGc3BhbiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZkaXYlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDdHIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGRhdGEtdGglM0QlMjJDYXRhbG9nJTIwSUQlMjMlMjIlMjBjbGFzcyUzRCUyMmNhdGFsb2clMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NkaXYlMjBjbGFzcyUzRCUyMnByb2R1Y3QtaXRlbS1tb2RlbCUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2xhYmVsJTNFQVNDLTkwMjEtMyUzQyUyRmxhYmVsJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDc3BhbiUzRSUyMCUzQyUyRnNwYW4lM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZkaXYlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3RkJTIwZGF0YS10aCUzRCUyMlByb2R1Y3QlMjBOYW1lJTIyJTIwY2xhc3MlM0QlMjJwcm9kdWN0LW5hbWUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NkaXYlMjBjbGFzcyUzRCUyMnByb2R1Y3QlMjBuYW1lJTIwcHJvZHVjdC1pdGVtLW5hbWUlMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0NhJTIwY2xhc3MlM0QlMjJwcm9kdWN0LWl0ZW0tbGluayUyMiUyMGhyZWYlM0QlMjJodHRwcyUzQSUyRiUyRnd3dy5hcHBsaWVkc3RlbWNlbGwuY29tJTJGcmVzZWFyY2glMkZzZXJ2aWNlcyUyRnN0ZW0tY2VsbC1zZXJ2aWNlcyUyRnN0ZW0tY2VsbC1nZW5lcmF0aW9uJTJGYXNjLTkwMjEtMy10cmktbGluZWFnZS1kaXJlY3RlZC1kaWZmZXJlbnRpYXRpb24lMjIlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjBUcmktTGluZWFnZSUyMERpcmVjdGVkJTIwRGlmZmVyZW50aWF0aW9uJTIwJTI4Zm9yJTIwaVBTQyUyMDMlMjBjbG9uZXMlMjklMjBTZXJ2aWNlJTIwZnJvbSUyMGlQU0MlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZhJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGZGl2JTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGQlM0UlMEElMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0N0ZCUyMGRhdGEtdGglM0QlMjJQcmljZSUyMiUyMGNvbHNwYW4lM0QlMjIyJTIyJTIwY2xhc3MlM0QlMjJwcm9kdWN0LXByaWNlJTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDZGl2JTIwY2xhc3MlM0QlMjJwcm9kdWN0LWl0ZW0taW5xdWVyeSUyMGFjdGlvbnMtcHJpbWFyeSUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2ElMjBocmVmJTNEJTIyJTJGY29udGFjdHVzJTJGaW5kZXglMkYlM0ZpZCUzRDQyODMlMjZhbXAlM0JjYXRhbG9nJTNEQVNDLTkwMjEtMyUyMiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ3NwYW4lM0VJbnF1aXJlJTNDJTJGc3BhbiUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRmElM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZkaXYlM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0ZCUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQyUyRnRyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDJTJGdGJvZHklM0UlMEElMjAlMjAlMjAlMjAlMjAlMjAlMjAlMjAlM0MlMkZ0YWJsZSUzRQ==[/vc_raw_html][/vc_column_inner][/vc_row_inner][/vc_column][/vc_row][vc_row el_wrapper_class=”services-accordion-box”][vc_column][vc_tta_accordion][vc_tta_section title=”Publications” tab_id=”1714253693865-4168b561-226e”][vc_raw_html css=””]JTNDdWwlM0UlMEElM0NsaSUzRUphbmclMkMlMjBZLiUyQyUyMENob2klMkMlMjBKLiUyQyUyMFBhcmslMkMlMjBOLiUyQyUyMEthbmclMkMlMjBKLiUyQyUyMEtpbSUyQyUyME0uJTJDJTIwS2ltJTJDJTIwWS4lMkMlMjAlMjZhbXAlM0IlMjBKdSUyQyUyMEouJTIwSC4lMjAlMjgyMDE5JTI5LiUyMERldmVsb3BtZW50JTIwb2YlMjBpbW11bm9jb21wYXRpYmxlJTIwcGx1cmlwb3RlbnQlMjBzdGVtJTIwY2VsbHMlMjB2aWElMjBDUklTUFItYmFzZWQlMjBodW1hbiUyMGxldWtvY3l0ZSUyMGFudGlnZW4lMjBlbmdpbmVlcmluZy4lMjZuYnNwJTNCJTNDZW0lM0UlM0NhJTIwaHJlZiUzRCUyMmh0dHBzJTNBJTJGJTJGd3d3Lm5hdHVyZS5jb20lMkZhcnRpY2xlcyUyRnMxMjI3Ni0wMTgtMDE5MC0yJTIyJTIwdGFyZ2V0JTNEJTIyX2JsYW5rJTIyJTIwcmVsJTNEJTIybm9vcGVuZXIlMjIlM0VFeHBlcmltZW50YWwlMjAlMjZhbXAlM0IlMjBNb2xlY3VsYXIlMjBNZWRpY2luZSUyQyUyNm5ic3AlM0I1MSUyODElMjklMkMlMjAzJTNDJTJGYSUzRSUzQyUyRmVtJTNFLiUzQyUyRmxpJTNFJTBBJTNDbGklM0VJbGljJTJDJTIwRC4lMjAlMjgyMDE5JTI5LiUyMExhdGVzdCUyMGRldmVsb3BtZW50cyUyMGluJTIwdGhlJTIwZmllbGQlMjBvZiUyMHN0ZW0lMjBjZWxsJTIwcmVzZWFyY2glMjBhbmQlMjByZWdlbmVyYXRpdmUlMjBtZWRpY2luZSUyMGNvbXBpbGVkJTIwZnJvbSUyMHB1YmxpY2x5JTIwYXZhaWxhYmxlJTIwaW5mb3JtYXRpb24lMjBhbmQlMjBwcmVzcyUyMHJlbGVhc2VzJTIwZnJvbSUyMG5vbmFjYWRlbWljJTIwaW5zdGl0dXRpb25zJTIwaW4lMjBPY3RvYmVyJTIwMjAxOC4lMjZuYnNwJTNCJTNDZW0lM0UlM0NhJTIwaHJlZiUzRCUyMmh0dHBzJTNBJTJGJTJGd3d3LmZ1dHVyZW1lZGljaW5lLmNvbSUyRmRvaSUyRmFicyUyRjEwLjIyMTclMkZybWUtMjAxOC0wMTQ3JTNGam91cm5hbENvZGUlM0RybWUlMjIlMjB0YXJnZXQlM0QlMjJfYmxhbmslMjIlMjByZWwlM0QlMjJub29wZW5lciUyMiUzRVJlZ2VuZXJhdGl2ZSUyMG1lZGljaW5lJTJDJTI2bmJzcCUzQjE0JTI4MiUyOSUyQyUyMDg1LTkyJTNDJTJGYSUzRSUzQyUyRmVtJTNFLiUzQyUyRmxpJTNFJTBBJTNDbGklM0VBbGxlbmRlJTJDJTIwTS4lMjBMLiUyQyUyMENvb2slMkMlMjBFLiUyMEsuJTJDJTIwTGFybWFuJTJDJTIwQi4lMjBDLiUyQyUyME51Z2VudCUyQyUyMEEuJTJDJTIwQnJhZHklMkMlMjBKLiUyME0uJTJDJTIwR29sZWJpb3dza2klMkMlMjBELiUyQyUyMC4uLiUyMCUyNmFtcCUzQiUyMFByb2lhJTJDJTIwUi4lMjBMLiUyMCUyODIwMTglMjkuJTIwQ2VyZWJyYWwlMjBvcmdhbm9pZHMlMjBkZXJpdmVkJTIwZnJvbSUyMFNhbmRob2ZmJTIwZGlzZWFzZSUyMGluZHVjZWQlMjBwbHVyaXBvdGVudCUyMHN0ZW0lMjBjZWxscyUyMGV4aGliaXQlMjBpbXBhaXJlZCUyMG5ldXJvZGlmZmVyZW50aWF0aW9uLiUzQ2ElMjBocmVmJTNEJTIyaHR0cCUzQSUyRiUyRnd3dy5qbHIub3JnJTJGY29udGVudCUyRmVhcmx5JTJGMjAxOCUyRjAxJTJGMjIlMkZqbHIuTTA4MTMyMy5mdWxsLnBkZiUyQmh0bWwlMjIlMjB0YXJnZXQlM0QlMjJfYmxhbmslMjIlMjByZWwlM0QlMjJub29wZW5lciUyMiUzRSUyNm5ic3AlM0IlM0NlbSUzRUpvdXJuYWwlMjBvZiUyMExpcGlkJTIwUmVzZWFyY2glM0MlMkZlbSUzRSUyQyUyMGpsci1NMDgxMzIzLiUzQyUyRmElM0UlM0MlMkZsaSUzRSUwQSUzQ2xpJTNFRmllbGQlMkMlMjBBLiUyMFIuJTJDJTIwSmFjb2JzJTJDJTIwRi4lMjBNLiUyQyUyMEZpZGRlcyUyQyUyMEkuJTIwVC4lMkMlMjBQaGlsbGlwcyUyQyUyMEEuJTIwUC4lMkMlMjBSZXllcy1PcnRpeiUyQyUyMEEuJTIwTS4lMkMlMjBMYU1vbnRhZ25lJTJDJTIwRS4lMkMlMjAuLi4lMjAlMjZhbXAlM0IlMjBIYXVlc3NsZXIlMkMlMjBNLiUyMCUyODIwMTklMjkuJTIwU3RydWN0dXJhbGx5JTIwQ29uc2VydmVkJTIwUHJpbWF0ZSUyMExuY1JOQXMlMjBBcmUlMjBUcmFuc2llbnRseSUyMEV4cHJlc3NlZCUyMGR1cmluZyUyMEh1bWFuJTIwQ29ydGljYWwlMjBEaWZmZXJlbnRpYXRpb24lMjBhbmQlMjBJbmZsdWVuY2UlMjBDZWxsLVR5cGUtU3BlY2lmaWMlMjBHZW5lcy4lMjZuYnNwJTNCJTNDZW0lM0UlM0NhJTIwaHJlZiUzRCUyMmh0dHBzJTNBJTJGJTJGd3d3LnNjaWVuY2VkaXJlY3QuY29tJTJGc2NpZW5jZSUyRmFydGljbGUlMkZwaWklMkZTMjIxMzY3MTExODMwNTI1MyUyMiUyMHRhcmdldCUzRCUyMl9ibGFuayUyMiUyMHJlbCUzRCUyMm5vb3BlbmVyJTIyJTNFU3RlbSUyMGNlbGwlMjByZXBvcnRzJTNDJTJGYSUzRSUzQyUyRmVtJTNFLiUzQyUyRmxpJTNFJTBBJTNDJTJGdWwlM0U=[/vc_raw_html][/vc_tta_section][/vc_tta_accordion][/vc_column][/vc_row][vc_row el_wrapper_class=”faq-accordion-box”][vc_column][vc_column_text css=””]
Frequently Asked Questions
[/vc_column_text][vc_tta_accordion][vc_tta_section title=”Do you receive patient whole blood samples for iPSC generation? Do we need to perform any pathogen tests prior to shipping the samples to us?” tab_id=”1714400285991-1ad3e6bb-b362f61a-1e804c7b-9f27″][vc_column_text css=””]We ask that you send in pathogen test results before you send the blood samples to us.
If you do not have pathogen test results, you can send the blood samples to us directly, and we will do the sample process and culture in a quarantine room for 1-2 weeks before we submit and get pathogen test results. A quarantine room fee is applied. Inquire for more details.[/vc_column_text][/vc_tta_section][/vc_tta_accordion][vc_tta_accordion active_section=””][vc_tta_section title=”What will customers need to provide for iPSC generation from patient fibroblasts?” tab_id=”1714400506975-b4326d2b-af18f61a-1e804c7b-9f27″][vc_column_text css=””]For iPSC generation from patient fibroblasts, customers will need to provide 1 x 10^6 cells.[/vc_column_text][/vc_tta_section][/vc_tta_accordion][vc_tta_accordion active_section=””][vc_tta_section title=”What is the minimum numbers of cells needed for iPSC generation from PBMCs?” tab_id=”1714553243310-d709a6fc-a78d”][vc_column_text css=””]For iPSC generation from PBMCs, we will need 2 vials of 2-3 x 10^6 cells.[/vc_column_text][/vc_tta_section][/vc_tta_accordion][vc_tta_accordion active_section=””][vc_tta_section title=”Would you be able to receive a skin biopsy for iPSC generation?” tab_id=”1714553262212-47e7cad7-f7c5″][vc_column_text css=””]Yes, we can receive skin biopsy. The skin biopsy can be stored immediately after the collection and shipped overnight in PBS or complete medium (DMEM/10% FBS) at 4°C.[/vc_column_text][/vc_tta_section][/vc_tta_accordion][vc_tta_accordion active_section=””][vc_tta_section title=”What are iPSCs?” tab_id=”1714553282609-c1aa1b14-8f00″][vc_column_text css=””]Induced pluripotent stem cells (iPSCs) are pluripotent cells that have been reprogrammed from adult somatic cells to an embryonic stem cell-like state. iPSCs can be genetically modified and further differentiated to committed somatic lineages.[/vc_column_text][/vc_tta_section][/vc_tta_accordion][vc_tta_accordion active_section=””][vc_tta_section title=”What are Yamanaka factors?” tab_id=”1714553299691-3113be98-59f4″][vc_column_text css=””]Yamanaka factors are four transcriptions (Oct3/4, Sox2, Klf4, and c-Myc) that can induce pluripotency. Induced pluripotent stem cells (iPSCs) can be generated from adult somatic cells by the introduction of the Yamanaka factors.[/vc_column_text][/vc_tta_section][/vc_tta_accordion][vc_tta_accordion active_section=””][vc_tta_section title=”Is it foot-print free?” tab_id=”1714553320620-a50e2154-5cb2″][vc_column_text css=””]ASC offers footprint-free reprogramming with feeder-free culture conditions.[/vc_column_text][/vc_tta_section][/vc_tta_accordion][vc_tta_accordion active_section=””][vc_tta_section title=”Any pathogen testing required?” tab_id=”1714553339145-45a071b7-efaf”][vc_column_text css=””]ASC’s custom iPSC generation service includes pathogen testing.[/vc_column_text][/vc_tta_section][/vc_tta_accordion][vc_tta_accordion active_section=””][vc_tta_section title=”What characterization is included?” tab_id=”1714553354459-45922027-c24f”][vc_column_text css=””]iPSCs characterized for morphology and pluripotency markers. Additional characterization such as G-banding, RT-PCR, STR profiling, directed differentiation is also available.[/vc_column_text][/vc_tta_section][/vc_tta_accordion][vc_tta_accordion active_section=””][vc_tta_section title=”How long does it take to reprogram iPSCs?” tab_id=”1714553373860-8e2f2cdd-c172″][vc_column_text css=””]Turnaround time: 2-3 months[/vc_column_text][/vc_tta_section][/vc_tta_accordion][vc_tta_accordion active_section=””][vc_tta_section title=”Can you generate iPSC feeder free?” tab_id=”1714553393273-7ebde4f3-66b5″][vc_column_text css=””]Yes, we use feeder-free protocols. Optional feeder-dependent protocols are available.[/vc_column_text][/vc_tta_section][/vc_tta_accordion][vc_tta_accordion active_section=””][vc_tta_section title=”How do you confirm pluripotency?” tab_id=”1714553415199-9f6ac9fd-3009″][vc_column_text css=””]iPSCs characterized for morphology and pluripotency markers (OCT4, SOX2, SSEA4, TRA-1-60, TRA-1-81). Additional characterization such as G-banding, RT-PCR, STR profiling, directed differentiation is also available.[/vc_column_text][/vc_tta_section][/vc_tta_accordion][/vc_column][/vc_row][vc_row padding_top_multiplier=”2x” padding_bottom_multiplier=”2x”][vc_column width=”1/2″ el_id=”custom-learn-more-img”][movedo_single_image image=”3181″ el_class=”custom-learn-more-img”][vc_custom_heading text=”Learn More” font_container=”tag:p|font_size:40px|text_align:center|line_height:60px” use_theme_fonts=”yes” css=”” el_id=”custom-learn-more-heading”][/vc_column][vc_column width=”1/2″][vc_raw_html css=””]JTNDZGl2JTIwaWQlM0QlMjJjdXN0b20tbGVhcm4tbW9yZS1yaWdodCUyMiUzRSUwQSUyMCUyMCUyMCUzQ3VsJTIwaWQlM0QlMjJjdXN0b20tbGVhcm4tbW9yZS1saXN0JTIyJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDbGklMjBjbGFzcyUzRCUyMmxpc3QtaXRlbSUyMiUzRSUzQ2ElMjBocmVmJTNEJTIyaHR0cHMlM0ElMkYlMkZwaW5jaGZvcnRodGVzdGluZy5jb20lMkZpcHNjLWRpZmZlcmVudGlhdGlvbiUyRiUyMiUzRSUzQ2ltZyUyMHNyYyUzRCUyMmh0dHBzJTNBJTJGJTJGcGluY2hmb3J0aHRlc3RpbmcuY29tJTJGd3AtY29udGVudCUyRnVwbG9hZHMlMkYyMDI0JTJGMDUlMkZhcnJvdy1jaXJjbGUtdXAtcmlnaHQucG5nJTIyJTIwYWx0JTNEJTIySW1hZ2UlMjAyJTIyJTNFaVBTQyUyMERpZmZlcmVudGlhdGlvbiUzQyUyRmElM0UlM0MlMkZsaSUzRSUwQSUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUyMCUzQ2xpJTIwY2xhc3MlM0QlMjJsaXN0LWl0ZW0lMjIlM0UlM0NhJTIwaHJlZiUzRCUyMmh0dHBzJTNBJTJGJTJGcGluY2hmb3J0aHRlc3RpbmcuY29tJTJGaXBzYy1nZW5vbWUtZWRpdGluZyUyRiUyMiUzRSUzQ2ltZyUyMHNyYyUzRCUyMmh0dHBzJTNBJTJGJTJGcGluY2hmb3J0aHRlc3RpbmcuY29tJTJGd3AtY29udGVudCUyRnVwbG9hZHMlMkYyMDI0JTJGMDUlMkZhcnJvdy1jaXJjbGUtdXAtcmlnaHQucG5nJTIyJTIwYWx0JTNEJTIySW1hZ2UlMjAyJTIyJTNFaVBTQyUyMEdlbmUlMjBFZGl0aW5nJTNDJTJGYSUzRSUzQyUyRmxpJTNFJTBBJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTIwJTNDbGklMjBjbGFzcyUzRCUyMmxpc3QtaXRlbSUyMiUzRSUzQ2ElMjBocmVmJTNEJTIyaHR0cHMlM0ElMkYlMkZwaW5jaGZvcnRodGVzdGluZy5jb20lMkZnbXAtaXBzYyUyRiUyMiUzRSUzQ2ltZyUyMHNyYyUzRCUyMmh0dHBzJTNBJTJGJTJGcGluY2hmb3J0aHRlc3RpbmcuY29tJTJGd3AtY29udGVudCUyRnVwbG9hZHMlMkYyMDI0JTJGMDUlMkZhcnJvdy1jaXJjbGUtdXAtcmlnaHQucG5nJTIyJTIwYWx0JTNEJTIySW1hZ2UlMjAzJTIyJTNFR01QJTIwaVBTQyUzQyUyRmElM0UlM0MlMkZsaSUzRSUwQSUyMCUyMCUyMCUzQyUyRnVsJTNFJTBBJTNDJTJGZGl2JTNF[/vc_raw_html][/vc_column][/vc_row]